After paying out $56.5 million as part of the settlement with federal and state governments in 2014 for allegedly misleading the public, US and state agencies by claiming Adderall would ‘normalize’ its users and Vyvanse would provide ‘less abuse liability’, a former executive from Shire Pharmaceuticals (now Takeda Pharmaceutical Company), in charge of Data Science, has taken legal action against the company. The whistleblower, Dr. Vincent Polito, alleges that Shire failed to comply with Drug Enforcement Administration mandates, such as monitoring and reporting suspicious drug orders for a certain period. The whistleblower asserts that his termination from the company resulted from repeatedly blowing the whistle and ultimately informing the company’s Chief Compliance Officer against the wishes of his management.
Dr. Polito claims that after being recruited from IBM in 2014, and after only several weeks on the job, he was requested by Shire execs to investigate and create a DEA-compliant suspicious order monitoring system (SOM) to monitor and report suspicious orders. Documents in the Court filings allege that several of Shire’s top execs created roadblocks to prevent him from completing his work. The whistleblower claims he was terminated from his project in mid-2015, after he and a consultant, along with the head of the SOM project, West Graham, visited Shire’s US Distribution facility in Lexington, Kentucky.
At the Distribution facility, Dr. Polito found drugs that were marked for destruction – which were contained in bottles marked 50% full as a default measurement – were not verified by either counting or weighing the pill bottles’ contents. He claims to have found that Shire’s internal company sales data did not match the data supplied to the DEA. The court filings state Dr. Polito reported his discovery to top execs, but, shortly after, was removed from the project.
In January 2016, the whistleblower alleges that over the objection of his supervisor, Walter Mullikin, a named defendant, reported the mismanagement of ADHD to the then Chief Compliance Officer, Jeffrey Rosenbaum, a named defendant, who had no knowledge of the issue. Shortly after that, the documents show Dr. Polito was forcibly placed on leave and was never allowed to return. He was terminated in August of 2016. According to a letter sent by Shire to the whistleblower, he was terminated for unprofessional conduct and for not cooperating in an internal investigation.
In April 2016, while on forced paid leave, Dr. Polito filed a qui tam suit, which later was rejected by the US Attorney’s Office in Washington DC, over concerns that the alleged violations were not covered by the False Claims Act. In August 2018, he filed this wrongful termination suit in Massachusetts Superior Court, alleging he was fired in violation of Public Policy for whistleblowing activities.
Shire’s defense was typical: According to the filings, Dr. Polito was a bad employee who wouldn’t cooperate with his supervisors.
So why is this whistleblower important? Well, the public’s health is at stake: At the time of the Complaint, Shire manufactured and distributed Adderall and Vyvanse. Considered amphetamines, both drugs are listed as Scheduled II controlled substances because they have a “high potential for abuse” and can cause “severe psychic or physical and or psychological dependence.” As we’ve noted in previous reports, the abuse of these drugs during Covid has quite literally been off the charts.
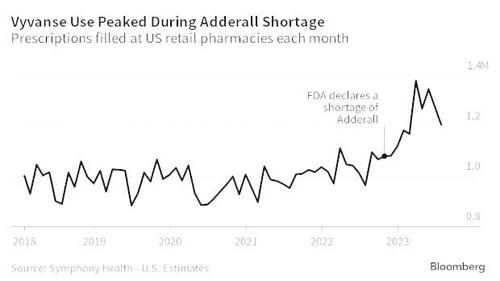
Under Federal law, Shire must maintain “effective control” against these drugs from falling into the wrong hands. For that reason, Shire and other organizations in the pharmaceutical industry are required under the Controlled Substances Act (CSA), to create internal automated systems to monitor and track suspicious orders of drugs like Adderall and Vyvanse and flag any orders of unusual size, unusual quantities or unusual purchasing patterns and report them to the DEA to protect the public’s health interest. The DEA relies on this requirement as the first line of defense against dishonest medical professionals who fuel the illegal market for these types of drugs.
Companies that fail to comply can have their distribution and/or manufacturing suspended and face massive fines. For example, in 2013 Walgreens agreed to pay a settlement of $80 million for similar violations. In 2015, McKesson Corp. agreed to a whopping $150 million for failing to report suspicious orders. In 2016, Cardinal Health, Inc., in a settlement with the Federal Government, paid $44 million for alleged violations. In 2017, manufacturer Mallinckrodt Pharmaceuticals settled its alleged violations for $35 million. And just this year after being accused of a delay, the DEA revoked one of the nation’s largest drug distributors Morris & Dickson Co. of its license for failing to flag suspicious opioid orders.
Corporate coverup?
In September 2014, as Dr. Polito began his work on Suspicious Order Monitoring, Shire agreed to pay $56.5 million as part of a settlement agreement with the Federal Government for alleged violations in their promotion of Adderall and Vyvanse. As part of the settlement, Shire entered into a five-year Corporate Integrity Agreement (CIA). According to Section III of this Agreement, Shire was required to establish and maintain a US Compliance Program. The Agreement requires the chief compliance officer to be responsible for monitoring the day-to-day compliance activities as well as reporting obligations under the CIA. The CIA was signed by then Chief Compliance Officer Caroline H. West.
According to court filings, Dr. Polito alleges that he tried to report his concerns to Ms. West by scheduling a meeting with her in July and August 2015. According to Polito’s sworn statement, after informing his boss, Walter Mullikin, the meeting never materialized. West left the company by the end of August of 2015. She was replaced by Jeffery Rosenbaum. According to sworn Declarations, in January 2016, when Polito reported the alleged SOM violations over the objections of his boss Mullikin, Rosenbaum, the new Chief Compliance Officer, had no clue as to the alleged violations and was never informed by his predecessor Caroline West – despite their responsibilities under the CIA.
Could the motivation by top execs to ‘cancel’ Dr. Polito revolve around mergers and acquisition activity?
From 2000 to the present, Shire has been involved in a number of mergers, acquisitions, and inversions. In November 2000, Shire and BioChem completed a $4 billion merger. Then, in April 2007, Shire acquired New River Pharmaceuticals for $2.6 billion. Around the time of Dr. Polito’s employment at Shire, the company was engaged with Abbvie in a reverse acquisition for $54 billion. The deal collapsed, and Shire received $1.6 billion for Abbvie’s failed attempt. In February 2015, Shire acquired NPS Pharmaceuticals for $5.2 billion. By 2016, just as Shire terminated the whistleblower, Shire acquired Baxalta for $32 billion. Shire struck gold in 2019 when Takeda Pharmaceutical Company purchased it for $62 billion.
Canceling the whistleblower only suggests Shire’s execs were driven by acquisition and merger to increase value and thwarted any attempt of a negative event, such as possible mismanagement of its primary products, Adderall and Vyvanse that would’ve involved a hefty fine from the DEA for breaking compliance.
The Securities and Exchange Commission mandates that public companies disclose details about substantial ongoing lawsuits. A preliminary review of Shire’s 10-K filings from 2018 to 2021 makes no mention of Dr. Polito’s case. It remains the responsibility of the SEC to determine if a lawsuit, which accuses breaches of the DEA’s Suspicious Orders regulations and potentially subjects Shire to fines or even license revocation, is deemed significant.
The case was originally filed in August 2018, with an amended complaint filed in December 2018, and before filing an answer to the complaint, Shire, through their Attorneys King & Spaulding and Choate, failed to have the case thrown out via a Motion to Dismiss. Discovery ensued. This year, in early 2023, Shire filed a Motion for Summary Judgment. The Motion was heard on July 11, 2023. The case is in the hands of Judge Christopher K. Barry-Smith Middlesex County Superior Court in Massachusetts as Dr. Polito and Shire await a decision.
Loading…
https://www.zerohedge.com/markets/corporate-coverup-whistleblower-doctor-alleges-shire-pharma-neglected-adhd-drug-monitoring